Our science
AMFA-targeting technology for advanced medicine
Our partnership proposals concern the creation of new therapies for patients by addressing the drugs inside the cells and to a specific cell compartment, the lysosomes. NanoMedSyn has developed proprietary synthetic derivatives of mannose 6-phosphate, called AMFA, that bind the mannose 6-phosphate receptor (M6PR). M6PR is demonstrated as a major pathway for the targeting of proteins bearing natural M6P or AMFA to lysosomes.
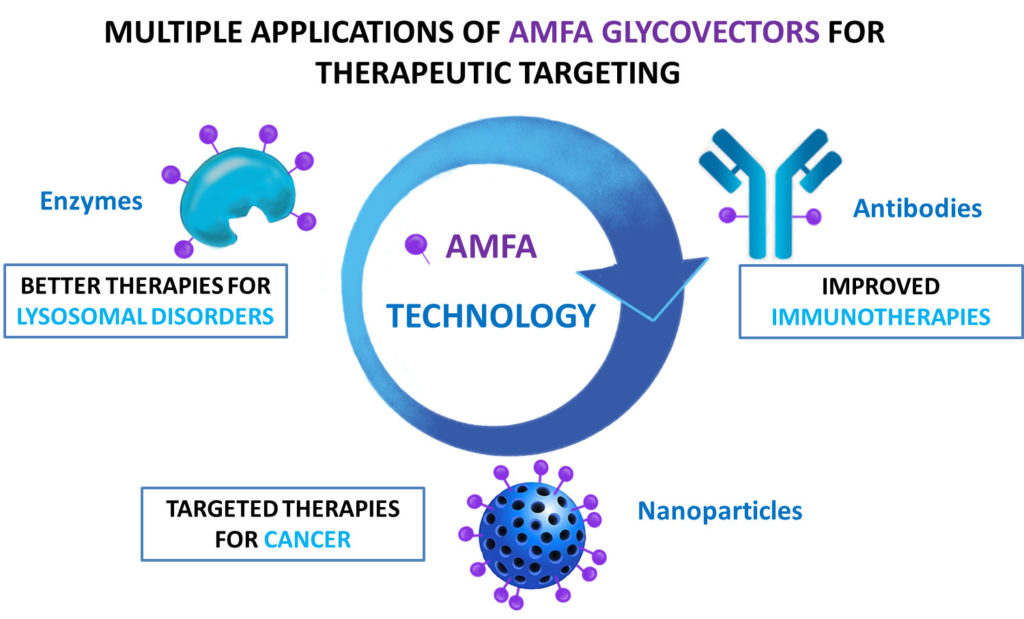
Our platform is quite versatile and can be applied to a number of therapeutic areas. The AMFA engineering is stable in vivo and insensitive to phosphatases in opposite to natural M6P. AMFA vectors can be conjugated to antibodies, enzymes or nanoparticles and offers a unique solution to unmet clinical needs.
Our team can perform AMFA-grafting and in vitro and in vivo characterisation of all types of AMFA-engineered drugs.
PARTNERSHIP PROPOSALS: From one platform to multiple therapeutic areas
- First-in-class therapeutic antibodies
Therapeutic antibodies engineered with AMFA acquire a higher cellular uptake and a new capacity to deplete specific soluble and membrane antigens by lysosomal degradation. AMFA-engineered antibodies preserve all desirable features of standard antibodies (recycling, immunogenicity, ADCC, etc) and AMFA-engineering is compatible with standard manufacturing processes.
This new class of AMFA-engineered antibodies is relevant to the treatment of autoimmune, inflammatory or cancer diseases.
- Enzyme replacement therapy of lysosomal storage diseases
NanoMedSyn has developed acid alpha-glucosidase conjugated with AMFA, aimed for the treatment of Pompe disease (1, 2 & 3). This AMFA engineered enzyme received an orphan drug designation by E.M.A. for enzyme replacement therapy of Pompe patients (4).
The benefits of AMFA mechanism of action on the enzyme processing up to the lysosome suggest a wider application to other lysosomal storage diseases and rare diseases (3).
- Therapeutic targeting of nanoparticles
The AMFA-engineering to nanoparticles has the capacity to increase their cellular uptake and their therapeutic efficacy in cancer cells (5, 6).
- Targeting prostate cancer and rhabdomyosarcoma
AMFA-grafting increase drug targeting to cancer cells overexpressing the M6PR as it is the case for prostate cancers (5) and rhabdomyosarcoma (6).